Enteromedics Inc.
Seite 1 von 4 Neuester Beitrag: 25.04.21 03:27 | ||||
| Eröffnet am: | 10.10.13 11:24 | von: Bäcker33 | Anzahl Beiträge: | 98 |
| Neuester Beitrag: | 25.04.21 03:27 | von: Jessikahypza | Leser gesamt: | 29.311 |
| Forum: | Hot-Stocks | Leser heute: | 10 | |
| Bewertet mit: | ||||
| Seite: < 1 | 2 | 3 | 4 4 > | ||||
http://www.streetinsider.com/Corporate+News/...+Obesity/10164301.html
It’s a tough hurdle. Many insurers and government agencies have refused to cover previous weight-loss treatments, such as Vivus Inc. (VVUS)’s Qsymia and Belviq, by Arena Pharmaceuticals Inc. and Eisai Co. To get a better outcome, EnteroMedics will have to show the device, Maestro Rechargeable System, is safe enough.
Insurance coverage could help EnteroMedics tap a market of 20 million obese patients in the U.S., according to Chief Financial Officer Greg Lea. The Food and Drug Administration approved the system today for obese patients 18 and older who have at least one other weight-related condition, such as Type 2 diabetes -- the first obesity device approved in the U.S. since 2007.
The FDA approval sent shares of EnteroMedics up 19 percent to $1.40 at the close, giving the company a market value of $97 million. The St. Paul, Minnesota-based company, founded in 2002, focuses on regulating the vagus nerve, which controls appetite.
Maestro is currently considered a Category 3 device by the FDA, with insufficient data to receive reimbursement by government agencies. EnteroMedics is pushing to have it classified as safe by next year, which would make private insurers more likely to come aboard, Lea said today in an interview.
Source: EnteroMedics Inc. via Bloomberg
EnteroMedics’ system uses electrodes implanted in the abdomen to send electrical pulses... Read More
“Our strength for the reimbursement path is, one, safety; we have a very strong benefit-versus-risk profile,” Lea said. “We don’t produce a lot of very severe issues with our procedure that create a million-dollar patient.”
Out of Pocket
In the meantime, EnteroMedics will try to introduce the treatment to patients who can pay out-of-pocket and don’t want to wait for insurance approval, Lea said. He estimated that the full price for Maestro, which targets the pathway between the brain and the stomach that controls feelings of hunger, could come in between $15,000 and $30,000. That’s competitive with comparable surgery options, according to Lea.
Maestro is likely to get insurance reimbursement because it fills an important gap, according to Chris Lewis, an analyst at Roth Capital Partners. He’s one of four who recommend buying the stock, while one analyst has a hold rating, according to data compiled by Bloomberg.
“There are a lot of patients in this gap between drugs and exercise and changes in diet, and on the other spectrum is the more invasive bariatric surgical options like Lap-Band,” Lewis said. “This fills that gap.”
Source: EnteroMedics Inc. via Bloomberg
EnteroMedics Inc.'s weight-loss device uses implantable electrodes to trick the brain... Read More
Getting government health agency approval by 2016 is still a “best-case scenario,” Lewis said.
“It’s not going to be a billion-dollar revenue company -- it’s going to take time to get there,” he said.
To contact the reporter on this story: Doni Bloomfield in New York at mbloomfiel12@bloomberg.net
To contact the editors responsible for this story: Crayton Harrison at tharrison5@bloomberg.net John Lear
EnteroMedics Announces Update on vBloc Therapy Trained Centers and Physicians
Fifteen Centers Certified and Nineteen Physicians Trained as of the end of the First Quarter of 2015
ST. PAUL, Minn., April 7, 2015 /PRNewswire/ -- EnteroMedics Inc. (NASDAQ: ETRM), the developer of medical devices using neuroblocking technology to treat obesity, metabolic diseases and other gastrointestinal disorders, today announced that 15 centers have been certified and 19 physicians were trained in implanting and administering vBloc Therapy as of the end of the first quarter of 2015, supporting the company's goal of training 20-25 vBloc Therapy centers and physicians by the end of 2015.
"Since receiving approval for vBloc Therapy in mid-January, we have been focused on identifying and training centers and physicians in key self-pay markets then working to integrate vBloc Therapy into their accounting and quality-assurance systems, a process that can take 2-3 months per center," said Brad Hancock, Chief Commercial Officer of EnteroMedics. "In parallel, we have been developing materials and programs to support patient access to vBloc Therapy, including a partnership with a healthcare lending company, American HealthCare Lending, as well as hosting well-attended patient webinars to educate patients on vBloc Therapy and building resources to help patients navigate their insurance programs. These efforts have put us on track to begin U.S. commercial implants this quarter."
EnteroMedics' bariatric center selection, training and certification process follows a defined protocol that includes rigorous center qualification criteria and didactic and surgical training. Once the center criteria are met, the Company's field staff trains the surgeon and staff on vBloc Therapy, theory of operation, and program implementation. This is followed by procedure and system operation training and one or two supervised surgeries, after which the surgeon is certified.
"The strong reception of vBloc Therapy by patients, centers and physicians has reinforced our belief that this first-of-its-kind treatment option fills the gap that exists between behavior modification or pharmaceutical options and anatomy altering and restricting surgery," said Mark B. Knudson, PhD, President and CEO of EnteroMedics. "We are pleased with the progress we have made with qualifying and training centers in the first quarter. We believe we have a solid pipeline of patients, physicians and centers to support our goals during this initial, controlled phase of our commercialization rollout in 2015 and look forward to providing additional updates on our progress as the year unfolds."
If you are interested in learning more about vBloc Therapy, please visit our physician locator at http://enteromedics.com/vbloc or call 1-800-MY-VBLOC.
EnteroMedics Announces First US Commercial Implant of vBloc® Neurometabolic Therapy System at Tufts Medical Center in Boston, Massachusetts
EnteroMedics Inc. (NASDAQ: ETRM), the developer of medical devices using neuroblocking technology to treat obesity, metabolic diseases and other gastrointestinal disorders, today announced the first ever commercial implant of the Maestro® Rechargeable System, delivering...
Posted By: activiststocksPosted date: May 27, 2015 10:04:12 PMIn: StocksNo Comments
Kevin Douglas owns 14.2% of the $100mm market cap medical device company. He’s owned shares for over half a decade, but recently decided to go active, here’s why:
The Filers acquired their shares of Common Stock for investment purposes because
they believed the Common Stock represented an attractive investment. However, they may at any time and from time to time determine to seek to contact the Issuer regarding means of increasing stockholder value.
Prior to the date hereof, the Filers had on file with the Securities and Exchange Commission a Schedule 13G with respect to their beneficial ownership of Common Stock, initially filed with the SEC on December 17, 2010. The Filers are filing this Schedule 13D in order to remove any possible impediment to contacting the Issuer regarding means of increasing stockholder value.
Werde mich mal weiter hineinlesen. Nur soviel vorweg:
Wenn das Produkt namens Maestro wirklich hält was es verspricht (und das tut es wohl), dann könnte ETRM eine neue Ära einläuten, in der es nicht nur weniger Fettleibige gibt, sondern damit verbunden auch weniger Herzpatienten, weniger Diabeteserkrankungen, u.s.w.
Sprich ein Schrecken für all die Pharmaunternehmen, die bisher ziemlich gut an der Medikation der Folgeerkrankungen verdient haben.
VG
EnteroMedics Announces the Launch of vBloc® Access
Program Designed to Support Patient Access to vBloc Neurometabolic Therapy
ST. PAUL, Minn., July 27, 2015 /PRNewswire/ -- EnteroMedics Inc. (NASDAQ: ETRM), the developer of medical devices using neuroblocking therapy to treat obesity, metabolic diseases and other gastrointestinal disorders, today announced the launch of vBloc® Access, a program designed to support patient access to vBloc Neurometabolic Therapy.
vBloc Access is a comprehensive patient support program that provides individuals considering vBloc Neurometabolic Therapy with help in understanding their insurance benefits and the insurance claim process. vBloc Access is available to physicians and healthcare providers as a resource for navigating the reimbursement process, including billing and coding assistance.
Case management experts are available to patients online at www.vbloc.com, and through a reimbursement support hotline at 1-866-77VBLOC (866-778-2562).
"EnteroMedics is pleased to offer patients and physicians with a resource to help them navigate the reimbursement process and provide expanded access to vBloc Neurometabolic Therapy," said Brad Hancock, EnteroMedics Chief Commercial Officer. "vBloc Neurometabolic Therapy is a unique product that enables healthy weight loss through a better patient experience. With the vBloc Access program, we look forward to supporting physicians and patients in meeting the rapidly growing interest in and demand for vBloc Neurometabolic Therapy."
http://ir.enteromedics.com/releasedetail.cfm?ReleaseID=923858
1. EnteroMedics to Host Second Quarter 2015 Financial Results and Company Update Conference Call
http://ir.enteromedics.com/releasedetail.cfm?ReleaseID=924708
http://ir.enteromedics.com/releasedetail.cfm?ReleaseID=925149
http://ir.enteromedics.com/releasedetail.cfm?ReleaseID=925818
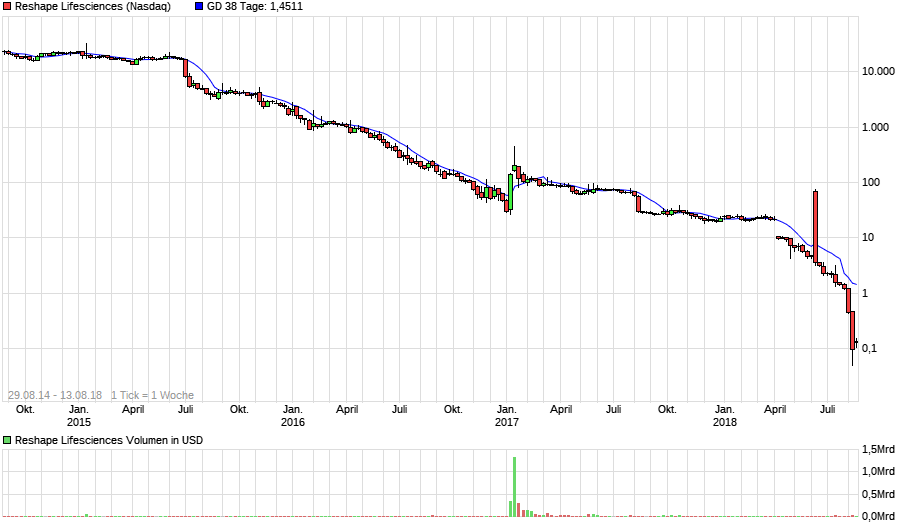
EnteroMedics Perf. seit Threadbeginn: -98,86%
Bottom fishing 0,1818 $